Qt Micro System, 0.22 Contamination tester, female luer lock inlet, male luer slip outlet, TSB Medi,
Item No.
TM6001
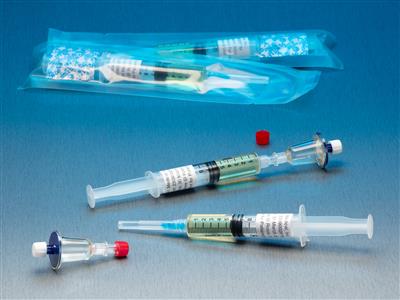
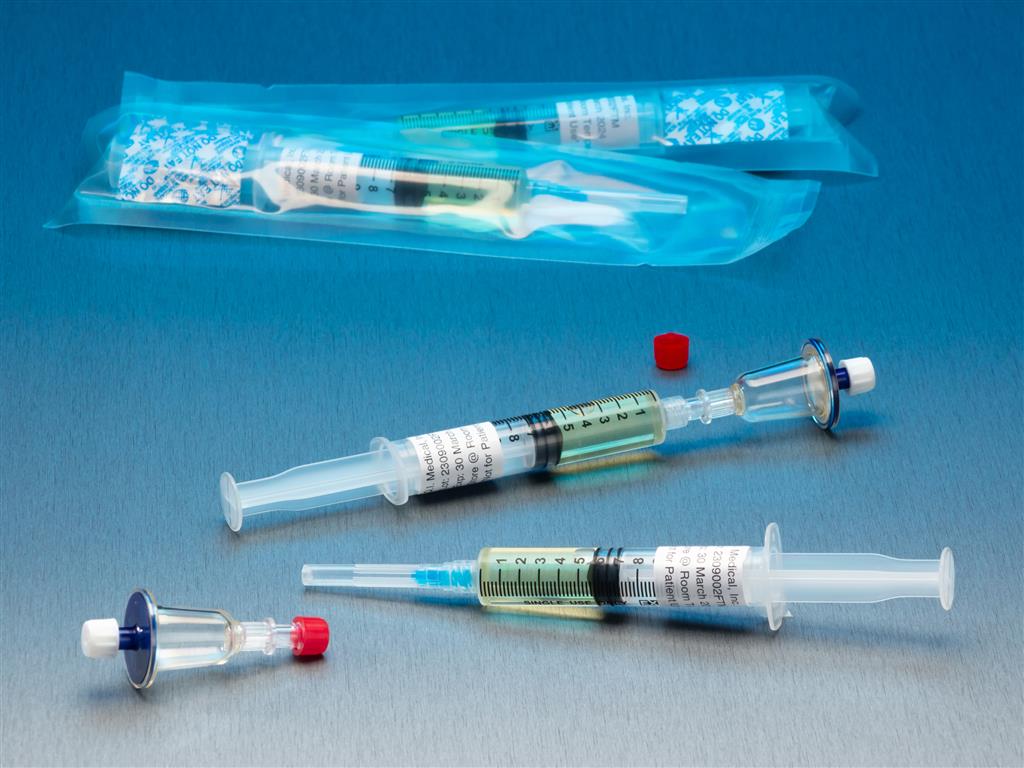
Qt Micro System Full filtration microbial contamination testing system for small volume compounded sterile products. Nondestructive test allows for sample to be used after testing. FDA Registered Class 2 Device with required FDA Unique Device Identifier (UDI) barcode. 0.22µ contamination tester for
Description
Qt Micro System
Full filtration microbial contamination testing system for small volume compounded sterile products.Nondestructive test allows for sample to be used after testing. FDA Registered Class 2 Device with required FDA Unique Device Identifier (UDI) barcode.
- 0.22µ contamination tester for syringe to syringe transfers.
- Log sheet included
- Gummed labels included
- (10) x QT Micro filter units with female luer lock inlet and female luer lock outlet.
- (10) x 5cc syringes of FTM growth media
Attachments
Reviews (0)
There are no reviews yet.