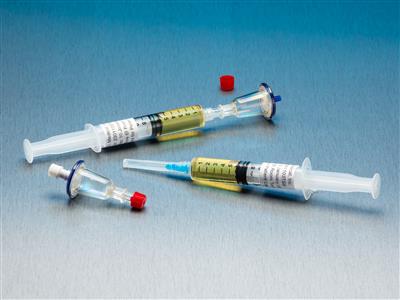
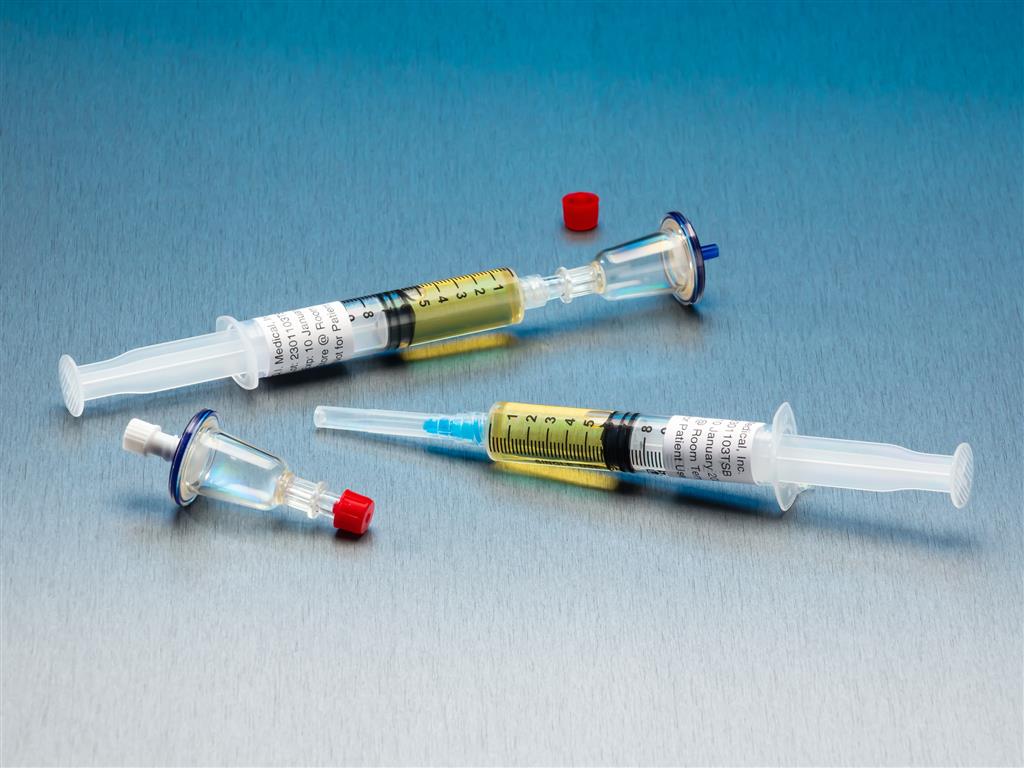
QT Micro 0.2 Micron Contamination Tester, 5mL TSB Syringe, Female LL Inlet, Male Luer Slip Outlet
Item No.
TM6100
QT Micro™ System Full filtration microbial testing system for small volume compounded sterile products. Non-destructive microbial test kit allows for sample to be used after testing. FDA Registered Class 2 Device with required FDA Unique Device Identifier (UDI) barcode. 0.22µ contamination tester
Description
QT Micro™ System
Full filtration microbial testing system for small volume compounded sterile products. Non-destructive microbial test kit allows for sample to be used after testing. FDA Registered Class 2 Device with required FDA Unique Device Identifier (UDI) barcode.- 0.22µ contamination tester for syringe to sterile transfers
- 10 x QT Micro filter units with female luer lock inlet and male luer slip outlet
- 10 x 5cc syringes of TSB growth media
- Log sheet included
- Gummed labels included
- 10 units per case
Attachments
Reviews (0)
There are no reviews yet.